Article from Cloud-based Medicine Studio | A national authoritative medical organization for rare diseases will be established, and the second list of rare diseases will be updated
(attachments: the first list and the national reimbursement drug list)
Published in Guangdong by Cloud-based Medicine Studio
Editor: Zifeiyu
Recently, the National Health Commission replied to the Proposal on Increasing Investment in the Diagnosis and Treatment of Rare Diseases, mentioning that the National Health Commission will update the second list of rare diseases when appropriate in accordance with the rare diseases listing procedures, and that the Chinese Medical Association plans to establish the first national authoritative medical organization for rare diseases, namely the Rare Diseases Branch of the Chinese Medical Association.
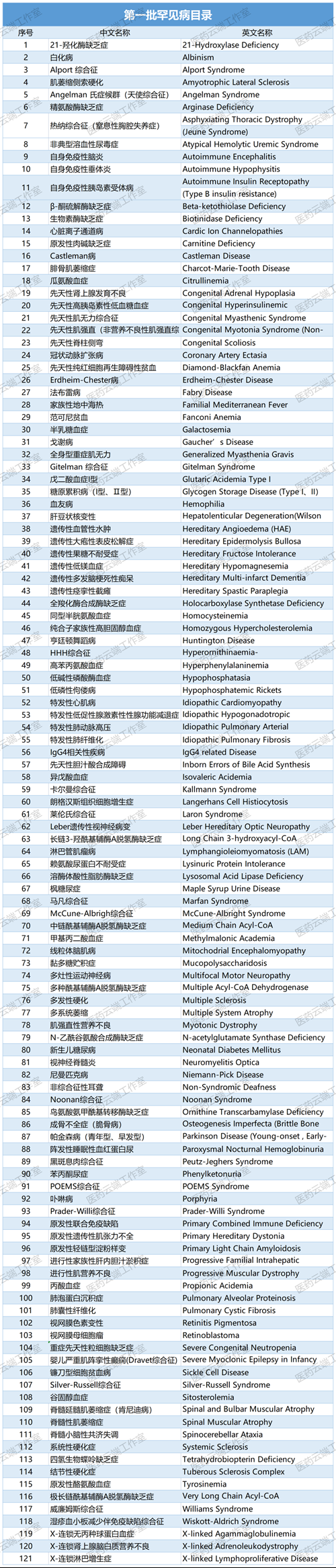
In May 2018, the National Health Commission, together with the Ministry of Science and Technology, the Ministry of Industry and Information Technology, and the National Medical Products Administration, released China’s First List of Rare Diseases, which included 121 rare diseases, providing an important basis for authorities to formulate policies related to rare diseases.
The next year, the National Health Commission formulated and issued the Guidelines for the Diagnosis and Treatment of Rare Diseases (covering each of the 121 rare diseases), and organized medical personnel training sessions through industry organizations.
In addition, through the Office of the National Collaborative Network for Diagnosis and Treatment of Rare Diseases, the National Health Commission provided doctor training for hospitals in the collaborative network to improve doctors’ abilities to diagnose and treat rare diseases.
According to the reply, the National Health Commission will update the list of rare diseases when appropriate in accordance with the rare diseases listing procedures, and carry out the training for the diagnosis and treatment of rare diseases through the Expert Committee on Rare Diseases Diagnosis, Treatment and Security and the National Collaborative Network for Diagnosis and Treatment of Rare Diseases.
Besides, the reply said that so far, some provinces and municipalities have established rare diseases-related expert committees, societies or study groups under local medical associations. The Chinese Medical Association is proactively preparing for the setup of the Rare Diseases Chapter of the Chinese Medical Association to play the role of a national authoritative academic organization.
According to Frost & Sullivan’s prediction, the Chinese market of drugs for rare diseases will grow to USD 25.9 billion in 2030. So far, more than 60 drugs for rare diseases have been approved for marketing in China, and more than 40 have been included in the national reimbursement drug list. With the continuous promulgation of favorable national policies, more Chinese enterprises are anticipated to engage in the R&D of drugs for rare diseases.
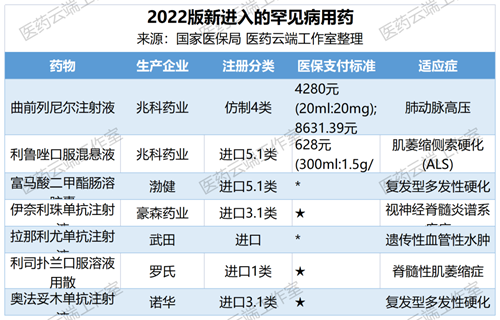
In this year’s adjustment of the national reimbursement drug list, more attention was paid to drugs for rare diseases, and 7 such drugs were finally included, covering multiple sclerosis, neuromyelitis optica and other rare diseases, which will significantly enhance China’s medical security for rare diseases and benefit patients with rare diseases.
About CANbridge Pharmaceuticals Inc.
CANbridge Pharmaceuticals Inc. (HKEX:1228) global biopharmaceutical company, with a foundation in China, committed to the research, development and commercialization of transformative therapies for rare disease and rare oncology. CANbridge has a differentiated drug portfolio, with three approved drugs and a pipeline of 10 assets, targeting prevalent rare disease and rare oncology indications that have unmet needs and significant market potential. These include Hunter syndrome and other lysosomal storage disorders, complement-mediated disorders, hemophilia A, metabolic disorders, rare cholestatic liver diseases and neuromuscular diseases, as well as glioblastoma multiforme. The CANbridge Next-Generation Innovation and Process Development Facility is developing novel, potentially curative, gene therapies for rare genetic diseases, including Pompe disease, Fabry disease, spinal muscular atrophy (SMA) and other neuromuscular conditions, and collaborates with world-leading researchers and biotech companies. Animal data from the SMA gene therapy was presented in 2022 at the American Society for Gene and Cell Therapy (ASGCT), the European Society for Gene and Cell Therapy (ESGCT) and the World Muscle Congress. CANbridge global partners include: Apogenix, GC Pharma, Mirum, Wuxi Biologics, Privus, UMass Chan Medical School, the University of Washington School of Medicine and Scriptr Global.
For more on CANbridge Pharmaceuticals Inc., please go to: www.canbridgepharma.com.
Forward-Looking Statements
The forward-looking statements made in this article relate only to the events or information as of the date on which the statements are made in this article. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the data on which the statements are made or to reflect the occurrence of unanticipated events. You should read this article completely and with the understanding that our actual future results or performance may be materially different from what we expect. In this article, statements of, or references to, our intentions or those of any of our Directors or our Company are made as of the date of this article. Any of these intentions may alter in light of future development.
Contact:
U.S. Investor Relations:
Chris Brinzey
ICR Westwicke
China Investor Relations
CANbridge Pharmaceuticals Inc.
Media:
Deanne Eagle
Planet Communications
deanne@planetcommunications.nyc
917.837.5866

 Back to Press Release
Back to Press Release
 Previous
Previous
